
P orbitals are orthogonal to one another which prevents the formation of pi bonds between each to the same atom. In addition, in order to rotate an atom about a double bond, the pi bonds must break first. This means that the third of the p orbitals is parallel to a given sigma bond and thus the atom will require invoking the d orbital in order to form quadruple bonds. As you can tell from the picture, only 2 of the 3 p orbitals can be perpendicular to the σ bond. Unlike normal sigma bonds which can freely rotate, the addition of pi bonds prevents a bond from rotating. It is important to note that their orientation is orthogonal, as well as being displaced somewhat, to the initial sigma bond and parallel to the p orbital of the other atom. The overlapping of un-hybridized filled p orbitals form π bonds. The different possible orientations (before pi bond formation) are also called “rotamers”, though sometimes they go by the name “ geometric isomers“.

This means that the groups attached to the atoms participating in the pi bond have a constant relative orientation. Due to the displacement of the pi electron cloud, any change in orientation between the atoms decreases orbital overlap.
#C2f6 sigma and pi bonds free#
However, once a pi bond forms between two atoms, all free rotation ceases. Ethane has free rotation around its pi bond, allowing for an infinite number of rotamers.
#C2f6 sigma and pi bonds full#
Often this rotation can occur very rapidly ethane, for instance, completes 10 10 full rotations around its C-C bond per second. Chemists call the different orientations these atoms can have with respect to one another rotamers, which have a lot of importance in organic chemistry.

Single bonds allow this because they only have one bond, a sigma bond, which connects the two nuclei precisely along their axis. This means that each atom in a single bond can rotate independent of one another.
#C2f6 sigma and pi bonds for free#
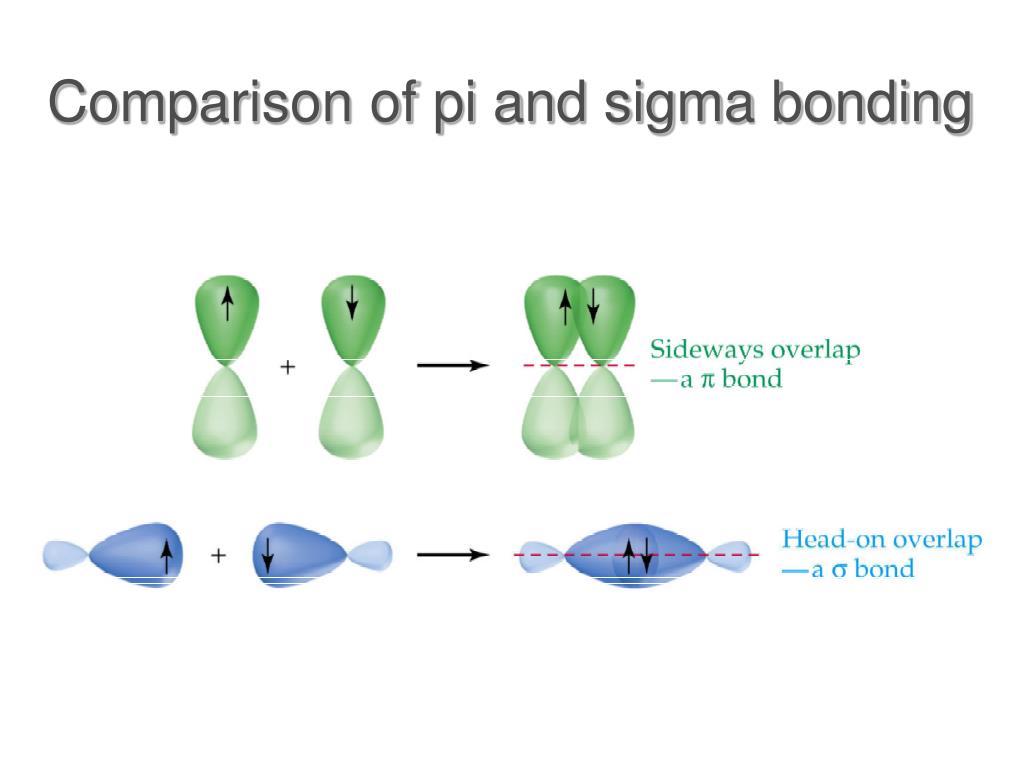
Specifically, single bonds allow for free rotation around the axis of the two nuclei. This has important ramifications for molecule in question with pi bonds. Pi Bonds and RotamersĪs you may notice, pi bonds form combined electron clouds that are a little displaced from the axis between the two nuclei. Referring to a molecule like diatomic nitrogen, the formation of N 2 would need 1 sigma and 2 pi bonds between the two nitrogen atoms to satisfy the triple bond. Thus to conclude, single bonds between atoms have a sigma bond any additional bonds between the two atoms are pi bonds double bonds have 1 sigma and 1 pi bond and triple bonds have 1 sigma and 2 pi bonds. Once this orbital is filled, the bond between the 2 atoms breaks. This bonding will also create an anti bonding orbital. This occurs when the un-hybridized (filled) p orbitals of each respective atom overlap to form a higher electron density between the two atoms outside of the established sigma bond. Different sigma and pi bond combinationsĪny additional bonds between two atoms are explained with the formation of pi bonds. The constructive interaction will thus lead to the formation of a sigma bond between the two atoms with a higher electron density shared between the two nuclei. Sigma bonds form when the available orbital with the highest energy of each atom overlaps one another. To start, we must explain both bonds: Sigma(σ) and Pi(π). Sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. You’ll also have the opportunity to apply what you know with some worked examples. In this article, you will learn about the basics of sigma and pi bonds and their importance to organic chemistry.


 0 kommentar(er)
0 kommentar(er)
